
Less well known than adult strokes are those occurring in
infants. They may affect up to
1,700 infants in the U.S. each year. Pediatric strokes are often related to changes in hemostasis in the mother or the placenta, particularly around the time of birth. An infant experiencing a stroke faces an
increased risk of long-term neurological disability, particularly cerebral palsy. Faced with possible life-long neurological damage, infants could benefit considerably from interventions that limit the cellular and tissue damage from these strokes when they happen. A promising candidate for reducing brain damage and improving outcomes associated with ischemia, hypoxia and neuronal damage is DHA, a long-chain (LC) n-3 PUFA. Encouraging results with DHA or n-3 LC-PUFAs have been reported in animal models of
traumatic brain injury,
spinal cord injury and
peripheral nerve damage. DHA or n-3 LC-PUFAs have also been associated with significantly
reduced axonal damage after head injury and with
reduced inflammation following spinal cord injury. In animal models of
stroke, DHA complexed to albumin has also been associated with neuroprotective benefits when administered up to 7 hours after the onset of stroke. Lipid mediators derived from DHA, such as neuroprotectin D1, have also been reported to promote
cell survival and sustain homeostasis in brain injury. These studies and others suggest that DHA might limit the damage from stroke and promote cell survival in pediatric patients. With the goal of curbing cerebral damage, investigators at Columbia University, New York, and at several pediatric and neuroscience research centers in the U.S. collaborated to study the effect of administering DHA-containing triglycerides to neonatal mice with hypoxia/ischemia brain injury. This
animal model has been well characterized and used as a
model for human neonatal hypoxia/ischemia brain injury. The investigators assessed the effects of triglyceride emulsions containing 55% linoleic acid from soybean oil, n-3 LC-PUFAs (~58% EPA plus DHA) from fish oil, or those with either >99% pure DHA or EPA prepared in the laboratory. The pure EPA and DHA emulsions were similar in size to VLDL particles. Ten-day-old neonatal mice underwent cerebral ischemia by ligation of the right common carotid artery, followed by 1.5 hours of recovery with their dams. At that time, the animals were exposed to systemic hypoxia for 15 min in a hypoxic chamber containing 8% humidified oxygen. Twenty-four hours after reperfusion the animals were sacrificed and their brains removed for evaluation. Treatment with the n-3 fish oil or n-6 emulsion was given to groups of mice by intraperitoneal injection immediately after surgery and again at the end of the hypoxic exposure. Other groups of animals were treated post-operatively after exposure to hypoxia and again 1 hour after reperfusion. Delayed treatments were evaluated in yet other animals, with the first dose given 1, 2 or 4 hours after hypoxia and then again one hour after the first dose. Brain damage was evaluated after injury by measuring cerebral blood flow using laser Doppler flowmetry and assessing the size of tissue loss in
the injured hemisphere compared with the intact
contralateral side. The investigators also measured bleeding time after the injection of 2 doses of n-3 triglycerides 2 hours apart and assessed the plasma concentrations of the n-3 LC-PUFA triglycerides. A key observation was the significant almost 50% reduction in tissue death in the hypoxia/ischemia-injured animals treated with n-3-LC-PUFA triglyceride emulsions, compared with the saline controls. The infarct volumes in the two groups were 35.1 ± 5.1% for the saline-treated animals compared with 19.9 ± 4.4% in the n-3-treated animals (P = 0.02). This effect was attributable mainly to DHA. Animals treated with n-6-PUFA triglycerides had infarct volumes more than 50% greater than the saline-treated controls. Lesions occurred mainly in the cortical and subcortical regions on the contralateral side from the artery ligation. Treatment with n-3-LC-PUFA triglycerides was associated with the greatest protection in the subcortical region. When the investigators compared the infarct volumes in animals treated with either 0.1 or 0.375 g triglyceride/kg body wt of pure DHA- or EPA-triglyceride after the injury, they observed that infarct volumes were reduced by 48% and 55% with each dose of DHA-triglyceride, respectively, (Figure). There was no effect on infarct volume with the EPA-triglyceride. Delaying the administration of DHA-triglyceride for 2 hours did not affect the reduction in infarct volume, but a delay of 4 hours abolished the protective effect.

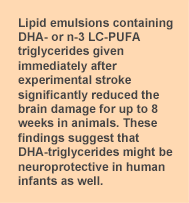
To learn whether the administration of DHA-triglyceride provided long-term neuroprotection, the investigators examined the brain tissue of DHA-triglyceride-treated injured animals 8 weeks after injury. They treated the animals with 0.375 g/kg body wt of DHA-triglyceride immediately after hypoxia/ischemia injury and one hour later. Tissue loss in the saline-treated controls was 25.0 ± 2.4% compared with 15.0 ± 2.5% in the DHA-triglyceride-treated animals (P = 0.02). Thus, a significant level of tissue protection was sustained 2 months after the hypoxia/ischemia injury. These studies demonstrated a significant reduction in brain tissue loss and infarct size from hypoxia/ischemia injury in neonatal mice treated with triglyceride emulsions containing n-3 LC-PUFAs, or pure DHA. EPA did not affect the amount of brain injury, while n-6 PUFAs increased the extent of tissue damage. DHA-triglyceride treatment led to reduced tissue loss when given up to 2 hours after injury, but not when delayed for 4 hours. Measurement of plasma triglyceride concentrations after injury showed a maximum increase 1.5 hours after the dose and a return to baseline levels by 3 hours, consistent with the pattern of n-3 LC-PUFA neuroprotection. Other investigators have reported that pretreatment with DHA prior to ischemic injury was associated with reduced brain infarction, inflammatory cell infiltration and other neuroprotective effects in
neonatal and
adult animals. The reduction in tissue damage was sustained at 8 weeks after the injury, when the DHA-triglyceride was given in 2 treatments immediately after injury. On the basis of preliminary unpublished studies in adult mice and results in a slightly different
stroke model in rats, the investigators suggested that the effectiveness of DHA-triglyceride could be related to the production of neuroprotectin D1 (NPD1) from DHA. Studies have reported that
NPD1 reduces inflammation, promotes the resolution of inflammatory responses, enhances cell survival, reduces apoptosis, protects synapses and signaling pathways and is involved in many activities that protect neurons and brain tissue from injury.
The study used parameters relevant to clinical stroke conditions in infants by using doses of triglyceride emulsions consistent with intravenous lipid emulsions used in human infants (1.5 g total triglyceride/kg body wt/day) and treatment times feasible in a neonatal intensive care unit. The studies defined a treatment window for effectiveness of approximately 2 hours after the injury. The authors noted that the differences in metabolic rate between mice and humans might permit a longer treatment window in humans, but this is conjecture. These findings buttress the evidence for DHA-triglycerides or n-3 LC-PUFAs as neuroprotective agents in stroke and cerebral injury and imply that their potential effectiveness in the treatment of pediatric stroke warrants further investigation. Williams JJ, Mayurasakorn K, Vannucci SJ, Mastropietro C, Bazan NG, Ten VS, Deckelbaum RJ. N-3 Fatty acid rich triglyceride emulsions are neuroprotective after cerebral hypoxic-ischemic injury in neonatal mice.
PLoS One 2013;8:e56233. [
PubMed] Open Access
Worth Noting Chang CL, Deckelbaum RJ. Omega-3 fatty acids: mechanisms underlying ‘protective effects’ in atherosclerosis.
Curr Opin Lipidol 2013;24:345-350. [
PubMed] de Lorgeril M, Salen P, Defaye P, Rabaeus M. Recent findings on the health effects of omega-3 fatty acids and statins, and their interactions: do statins inhibit omega-3?
BMC Med 2013;11:5-14. [
PubMed] Open Access de Goede J, Verschuren WM, Boer JM, Verberne LD, Kromhout D, Geleijnse JM. N-6 and n-3 fatty acid cholesteryl esters in relation to fatal CHD in a Dutch adult population: A nested case-control study and meta-analysis.
PLoS One 2013;8:e59408. [
PubMed] Open Access Otsuka R, Kato Y, Imai T, Ando F, Shimokata H. Higher serum EPA or DHA, and lower ARA compositions with age independent fatty acid intake in Japanese aged 40 to 79.
Lipids 2013;48:719-727. [
PubMed]
 Less well known than adult strokes are those occurring in infants. They may affect up to 1,700 infants in the U.S. each year. Pediatric strokes are often related to changes in hemostasis in the mother or the placenta, particularly around the time of birth. An infant experiencing a stroke faces an increased risk of long-term neurological disability, particularly cerebral palsy. Faced with possible life-long neurological damage, infants could benefit considerably from interventions that limit the cellular and tissue damage from these strokes when they happen. A promising candidate for reducing brain damage and improving outcomes associated with ischemia, hypoxia and neuronal damage is DHA, a long-chain (LC) n-3 PUFA. Encouraging results with DHA or n-3 LC-PUFAs have been reported in animal models of traumatic brain injury, spinal cord injury and peripheral nerve damage. DHA or n-3 LC-PUFAs have also been associated with significantly reduced axonal damage after head injury and with reduced inflammation following spinal cord injury. In animal models of stroke, DHA complexed to albumin has also been associated with neuroprotective benefits when administered up to 7 hours after the onset of stroke. Lipid mediators derived from DHA, such as neuroprotectin D1, have also been reported to promote cell survival and sustain homeostasis in brain injury. These studies and others suggest that DHA might limit the damage from stroke and promote cell survival in pediatric patients. With the goal of curbing cerebral damage, investigators at Columbia University, New York, and at several pediatric and neuroscience research centers in the U.S. collaborated to study the effect of administering DHA-containing triglycerides to neonatal mice with hypoxia/ischemia brain injury. This animal model has been well characterized and used as a model for human neonatal hypoxia/ischemia brain injury. The investigators assessed the effects of triglyceride emulsions containing 55% linoleic acid from soybean oil, n-3 LC-PUFAs (~58% EPA plus DHA) from fish oil, or those with either >99% pure DHA or EPA prepared in the laboratory. The pure EPA and DHA emulsions were similar in size to VLDL particles. Ten-day-old neonatal mice underwent cerebral ischemia by ligation of the right common carotid artery, followed by 1.5 hours of recovery with their dams. At that time, the animals were exposed to systemic hypoxia for 15 min in a hypoxic chamber containing 8% humidified oxygen. Twenty-four hours after reperfusion the animals were sacrificed and their brains removed for evaluation. Treatment with the n-3 fish oil or n-6 emulsion was given to groups of mice by intraperitoneal injection immediately after surgery and again at the end of the hypoxic exposure. Other groups of animals were treated post-operatively after exposure to hypoxia and again 1 hour after reperfusion. Delayed treatments were evaluated in yet other animals, with the first dose given 1, 2 or 4 hours after hypoxia and then again one hour after the first dose. Brain damage was evaluated after injury by measuring cerebral blood flow using laser Doppler flowmetry and assessing the size of tissue loss in
Less well known than adult strokes are those occurring in infants. They may affect up to 1,700 infants in the U.S. each year. Pediatric strokes are often related to changes in hemostasis in the mother or the placenta, particularly around the time of birth. An infant experiencing a stroke faces an increased risk of long-term neurological disability, particularly cerebral palsy. Faced with possible life-long neurological damage, infants could benefit considerably from interventions that limit the cellular and tissue damage from these strokes when they happen. A promising candidate for reducing brain damage and improving outcomes associated with ischemia, hypoxia and neuronal damage is DHA, a long-chain (LC) n-3 PUFA. Encouraging results with DHA or n-3 LC-PUFAs have been reported in animal models of traumatic brain injury, spinal cord injury and peripheral nerve damage. DHA or n-3 LC-PUFAs have also been associated with significantly reduced axonal damage after head injury and with reduced inflammation following spinal cord injury. In animal models of stroke, DHA complexed to albumin has also been associated with neuroprotective benefits when administered up to 7 hours after the onset of stroke. Lipid mediators derived from DHA, such as neuroprotectin D1, have also been reported to promote cell survival and sustain homeostasis in brain injury. These studies and others suggest that DHA might limit the damage from stroke and promote cell survival in pediatric patients. With the goal of curbing cerebral damage, investigators at Columbia University, New York, and at several pediatric and neuroscience research centers in the U.S. collaborated to study the effect of administering DHA-containing triglycerides to neonatal mice with hypoxia/ischemia brain injury. This animal model has been well characterized and used as a model for human neonatal hypoxia/ischemia brain injury. The investigators assessed the effects of triglyceride emulsions containing 55% linoleic acid from soybean oil, n-3 LC-PUFAs (~58% EPA plus DHA) from fish oil, or those with either >99% pure DHA or EPA prepared in the laboratory. The pure EPA and DHA emulsions were similar in size to VLDL particles. Ten-day-old neonatal mice underwent cerebral ischemia by ligation of the right common carotid artery, followed by 1.5 hours of recovery with their dams. At that time, the animals were exposed to systemic hypoxia for 15 min in a hypoxic chamber containing 8% humidified oxygen. Twenty-four hours after reperfusion the animals were sacrificed and their brains removed for evaluation. Treatment with the n-3 fish oil or n-6 emulsion was given to groups of mice by intraperitoneal injection immediately after surgery and again at the end of the hypoxic exposure. Other groups of animals were treated post-operatively after exposure to hypoxia and again 1 hour after reperfusion. Delayed treatments were evaluated in yet other animals, with the first dose given 1, 2 or 4 hours after hypoxia and then again one hour after the first dose. Brain damage was evaluated after injury by measuring cerebral blood flow using laser Doppler flowmetry and assessing the size of tissue loss in the injured hemisphere compared with the intact contralateral side. The investigators also measured bleeding time after the injection of 2 doses of n-3 triglycerides 2 hours apart and assessed the plasma concentrations of the n-3 LC-PUFA triglycerides. A key observation was the significant almost 50% reduction in tissue death in the hypoxia/ischemia-injured animals treated with n-3-LC-PUFA triglyceride emulsions, compared with the saline controls. The infarct volumes in the two groups were 35.1 ± 5.1% for the saline-treated animals compared with 19.9 ± 4.4% in the n-3-treated animals (P = 0.02). This effect was attributable mainly to DHA. Animals treated with n-6-PUFA triglycerides had infarct volumes more than 50% greater than the saline-treated controls. Lesions occurred mainly in the cortical and subcortical regions on the contralateral side from the artery ligation. Treatment with n-3-LC-PUFA triglycerides was associated with the greatest protection in the subcortical region. When the investigators compared the infarct volumes in animals treated with either 0.1 or 0.375 g triglyceride/kg body wt of pure DHA- or EPA-triglyceride after the injury, they observed that infarct volumes were reduced by 48% and 55% with each dose of DHA-triglyceride, respectively, (Figure). There was no effect on infarct volume with the EPA-triglyceride. Delaying the administration of DHA-triglyceride for 2 hours did not affect the reduction in infarct volume, but a delay of 4 hours abolished the protective effect.
the injured hemisphere compared with the intact contralateral side. The investigators also measured bleeding time after the injection of 2 doses of n-3 triglycerides 2 hours apart and assessed the plasma concentrations of the n-3 LC-PUFA triglycerides. A key observation was the significant almost 50% reduction in tissue death in the hypoxia/ischemia-injured animals treated with n-3-LC-PUFA triglyceride emulsions, compared with the saline controls. The infarct volumes in the two groups were 35.1 ± 5.1% for the saline-treated animals compared with 19.9 ± 4.4% in the n-3-treated animals (P = 0.02). This effect was attributable mainly to DHA. Animals treated with n-6-PUFA triglycerides had infarct volumes more than 50% greater than the saline-treated controls. Lesions occurred mainly in the cortical and subcortical regions on the contralateral side from the artery ligation. Treatment with n-3-LC-PUFA triglycerides was associated with the greatest protection in the subcortical region. When the investigators compared the infarct volumes in animals treated with either 0.1 or 0.375 g triglyceride/kg body wt of pure DHA- or EPA-triglyceride after the injury, they observed that infarct volumes were reduced by 48% and 55% with each dose of DHA-triglyceride, respectively, (Figure). There was no effect on infarct volume with the EPA-triglyceride. Delaying the administration of DHA-triglyceride for 2 hours did not affect the reduction in infarct volume, but a delay of 4 hours abolished the protective effect.  To learn whether the administration of DHA-triglyceride provided long-term neuroprotection, the investigators examined the brain tissue of DHA-triglyceride-treated injured animals 8 weeks after injury. They treated the animals with 0.375 g/kg body wt of DHA-triglyceride immediately after hypoxia/ischemia injury and one hour later. Tissue loss in the saline-treated controls was 25.0 ± 2.4% compared with 15.0 ± 2.5% in the DHA-triglyceride-treated animals (P = 0.02). Thus, a significant level of tissue protection was sustained 2 months after the hypoxia/ischemia injury. These studies demonstrated a significant reduction in brain tissue loss and infarct size from hypoxia/ischemia injury in neonatal mice treated with triglyceride emulsions containing n-3 LC-PUFAs, or pure DHA. EPA did not affect the amount of brain injury, while n-6 PUFAs increased the extent of tissue damage. DHA-triglyceride treatment led to reduced tissue loss when given up to 2 hours after injury, but not when delayed for 4 hours. Measurement of plasma triglyceride concentrations after injury showed a maximum increase 1.5 hours after the dose and a return to baseline levels by 3 hours, consistent with the pattern of n-3 LC-PUFA neuroprotection. Other investigators have reported that pretreatment with DHA prior to ischemic injury was associated with reduced brain infarction, inflammatory cell infiltration and other neuroprotective effects in neonatal and adult animals. The reduction in tissue damage was sustained at 8 weeks after the injury, when the DHA-triglyceride was given in 2 treatments immediately after injury. On the basis of preliminary unpublished studies in adult mice and results in a slightly different stroke model in rats, the investigators suggested that the effectiveness of DHA-triglyceride could be related to the production of neuroprotectin D1 (NPD1) from DHA. Studies have reported that NPD1 reduces inflammation, promotes the resolution of inflammatory responses, enhances cell survival, reduces apoptosis, protects synapses and signaling pathways and is involved in many activities that protect neurons and brain tissue from injury.
To learn whether the administration of DHA-triglyceride provided long-term neuroprotection, the investigators examined the brain tissue of DHA-triglyceride-treated injured animals 8 weeks after injury. They treated the animals with 0.375 g/kg body wt of DHA-triglyceride immediately after hypoxia/ischemia injury and one hour later. Tissue loss in the saline-treated controls was 25.0 ± 2.4% compared with 15.0 ± 2.5% in the DHA-triglyceride-treated animals (P = 0.02). Thus, a significant level of tissue protection was sustained 2 months after the hypoxia/ischemia injury. These studies demonstrated a significant reduction in brain tissue loss and infarct size from hypoxia/ischemia injury in neonatal mice treated with triglyceride emulsions containing n-3 LC-PUFAs, or pure DHA. EPA did not affect the amount of brain injury, while n-6 PUFAs increased the extent of tissue damage. DHA-triglyceride treatment led to reduced tissue loss when given up to 2 hours after injury, but not when delayed for 4 hours. Measurement of plasma triglyceride concentrations after injury showed a maximum increase 1.5 hours after the dose and a return to baseline levels by 3 hours, consistent with the pattern of n-3 LC-PUFA neuroprotection. Other investigators have reported that pretreatment with DHA prior to ischemic injury was associated with reduced brain infarction, inflammatory cell infiltration and other neuroprotective effects in neonatal and adult animals. The reduction in tissue damage was sustained at 8 weeks after the injury, when the DHA-triglyceride was given in 2 treatments immediately after injury. On the basis of preliminary unpublished studies in adult mice and results in a slightly different stroke model in rats, the investigators suggested that the effectiveness of DHA-triglyceride could be related to the production of neuroprotectin D1 (NPD1) from DHA. Studies have reported that NPD1 reduces inflammation, promotes the resolution of inflammatory responses, enhances cell survival, reduces apoptosis, protects synapses and signaling pathways and is involved in many activities that protect neurons and brain tissue from injury.  The study used parameters relevant to clinical stroke conditions in infants by using doses of triglyceride emulsions consistent with intravenous lipid emulsions used in human infants (1.5 g total triglyceride/kg body wt/day) and treatment times feasible in a neonatal intensive care unit. The studies defined a treatment window for effectiveness of approximately 2 hours after the injury. The authors noted that the differences in metabolic rate between mice and humans might permit a longer treatment window in humans, but this is conjecture. These findings buttress the evidence for DHA-triglycerides or n-3 LC-PUFAs as neuroprotective agents in stroke and cerebral injury and imply that their potential effectiveness in the treatment of pediatric stroke warrants further investigation. Williams JJ, Mayurasakorn K, Vannucci SJ, Mastropietro C, Bazan NG, Ten VS, Deckelbaum RJ. N-3 Fatty acid rich triglyceride emulsions are neuroprotective after cerebral hypoxic-ischemic injury in neonatal mice. PLoS One 2013;8:e56233. [PubMed] Open Access Worth Noting Chang CL, Deckelbaum RJ. Omega-3 fatty acids: mechanisms underlying ‘protective effects’ in atherosclerosis. Curr Opin Lipidol 2013;24:345-350. [PubMed] de Lorgeril M, Salen P, Defaye P, Rabaeus M. Recent findings on the health effects of omega-3 fatty acids and statins, and their interactions: do statins inhibit omega-3? BMC Med 2013;11:5-14. [PubMed] Open Access de Goede J, Verschuren WM, Boer JM, Verberne LD, Kromhout D, Geleijnse JM. N-6 and n-3 fatty acid cholesteryl esters in relation to fatal CHD in a Dutch adult population: A nested case-control study and meta-analysis. PLoS One 2013;8:e59408. [PubMed] Open Access Otsuka R, Kato Y, Imai T, Ando F, Shimokata H. Higher serum EPA or DHA, and lower ARA compositions with age independent fatty acid intake in Japanese aged 40 to 79. Lipids 2013;48:719-727. [PubMed]
The study used parameters relevant to clinical stroke conditions in infants by using doses of triglyceride emulsions consistent with intravenous lipid emulsions used in human infants (1.5 g total triglyceride/kg body wt/day) and treatment times feasible in a neonatal intensive care unit. The studies defined a treatment window for effectiveness of approximately 2 hours after the injury. The authors noted that the differences in metabolic rate between mice and humans might permit a longer treatment window in humans, but this is conjecture. These findings buttress the evidence for DHA-triglycerides or n-3 LC-PUFAs as neuroprotective agents in stroke and cerebral injury and imply that their potential effectiveness in the treatment of pediatric stroke warrants further investigation. Williams JJ, Mayurasakorn K, Vannucci SJ, Mastropietro C, Bazan NG, Ten VS, Deckelbaum RJ. N-3 Fatty acid rich triglyceride emulsions are neuroprotective after cerebral hypoxic-ischemic injury in neonatal mice. PLoS One 2013;8:e56233. [PubMed] Open Access Worth Noting Chang CL, Deckelbaum RJ. Omega-3 fatty acids: mechanisms underlying ‘protective effects’ in atherosclerosis. Curr Opin Lipidol 2013;24:345-350. [PubMed] de Lorgeril M, Salen P, Defaye P, Rabaeus M. Recent findings on the health effects of omega-3 fatty acids and statins, and their interactions: do statins inhibit omega-3? BMC Med 2013;11:5-14. [PubMed] Open Access de Goede J, Verschuren WM, Boer JM, Verberne LD, Kromhout D, Geleijnse JM. N-6 and n-3 fatty acid cholesteryl esters in relation to fatal CHD in a Dutch adult population: A nested case-control study and meta-analysis. PLoS One 2013;8:e59408. [PubMed] Open Access Otsuka R, Kato Y, Imai T, Ando F, Shimokata H. Higher serum EPA or DHA, and lower ARA compositions with age independent fatty acid intake in Japanese aged 40 to 79. Lipids 2013;48:719-727. [PubMed]

